Anaerobic Digestion of Animal Manures: Understanding the Basic Processes
The process of producing methane from manure is fairly straight forward – seal manure in an airtight container at a favorable temperature and it will break down into biogas: a mixture of Methane (CH4), Carbon dioxide (CO2), and trace amounts of other gases. Behind the apparent simplicity, though, lie complicated interactions involving several communities of microorganisms.
The Bioconversion Process (How Microbes Turn Organic Materials into Fuel)
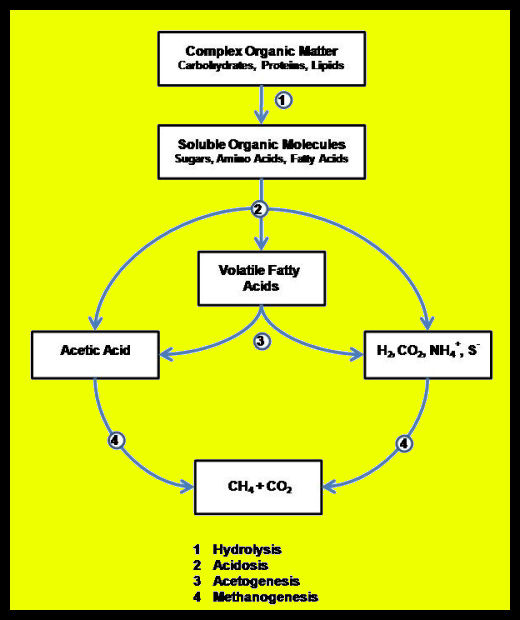
Anaerobic digestion is a multi-stage process (Figure 1) involving two to four steps, depending on where you want to draw lines in the process.
- Hydrolysis is the phase of anaerobic digestion where complex organic molecules are broken down into simpler organic molecules.
- Acidogenisis is where simple organic molecules are converted into fatty acids.
- Acetogenisis is where fatty acids are converted into acetate. This, along with acidogenisis, represents the transition from simple organic molecules to the methanogenic substrates as shown in the figure below.
- Methanogenisis is where molecules that have been converted into a suitable food source (or substrate) for methanogenic microorganisms are converted into methane by those organisms.
More than 100 different anaerobic microbes are known to contribute to the production of biogas. These microbes are organized into a number of interlinked communities. Communities of hydrolytic bacteria break complex organic matter down to simpler compounds. Acid-forming bacteria convert the simple compounds to volatile fatty acids – principally acetic acid (vinegar). Hydrolysis and acidogenisis are commonly lumped together and called anaerobic fermentation. Some microbiologists also distinguish between formation of mixed volatile fatty acids (acidosis) and reduction to Acetic acid (acetogenesis).
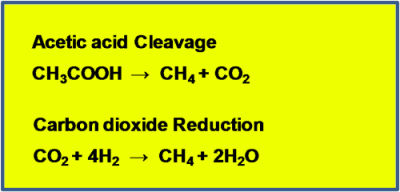
Methane forming Archaeans (very simple, single cell organisms similar to bacteria), called methanogens, take the end products of fermentation – volatile fatty acids, hydrogen gas (H2), CO2, and water (H2O) – and use them to form methane. Methanogens fall into two main camps depending on the pathway they use to produce methane (Figure 2). All methanogens can reduce CO2 and H2 into CH4 and H2O; those that use this pathway exclusively are called hydrotrophic methanogens. Methanogens that convert volatile fatty acids (and a number of other simple organic compounds) to CH4 and CO2 are called acetotrophic methanogens.
Limitations on Biological Processes
Anaerobic digestion is a living process, and like all biological communities, the microorganisms that carry out anaerobic fermentation and methanogensis do so under certain operating conditions.
Phases of Microbial Community Growth
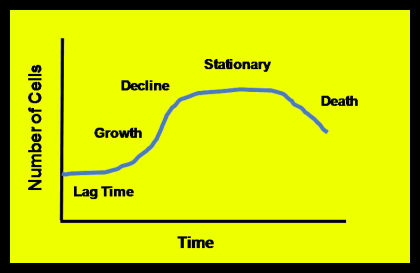
Given an ample food supply, sufficient room to expand, the absence of predators or competing organisms, all communities of organisms – be they methanogens or human beings – grow in a pattern similar to the one shown in Figure 3. The Lag Time occurs as the organisms acclimate to their environment.
- During the Growth Phase, food is not limiting, and population expands rapidly. Sometimes the Growth phase is called the Log Growth or Exponential Growth phase because the growth pattern follows an exponential curve. *Population growth slows down in the Decline Phase as the organisms meet the limit of their food supply.
- During the Stationary Phase, the community has met the limits of its food supply. Bear in mind that reproduction does not necessarily stop during the decline and stationary phases, only that the death rate approaches the reproduction rate. In some cases the community becomes dormant or goes into hibernation during the Stationary phase.
- Communities enter a Death or Endogenous Growth phase once a limited food supply is exhausted or an inhibiting element limits the further growth of organisms. During Endogenous Growth, the death rate exceeds the birth rate.
The inhibitory elements that cause endogenous growth are often the end products of a community’s metabolism.
The beauty of anaerobic digestion is that it is the work of a mixed community of organisms. The toxic end product of one community is the food supply of another. Acid forming bacteria consume the simple sugars that might inhibit hydrolytic communities. Methanogens use the acids formed in fermentation to produce CH4 and CO2. And in the end, CH4 and CO2 leave the digester as biogas.
Reproduction Time
Most anaerobic digesters are designed so that the microbial communities remain in exponential growth. An important concept to grasp is the doubling rate or reproduction time of an organism. This is the time needed for a population to double in size during exponential growth. In simple terms it is the time required for the organisms to replace themselves.
Hydraulic Retention Time
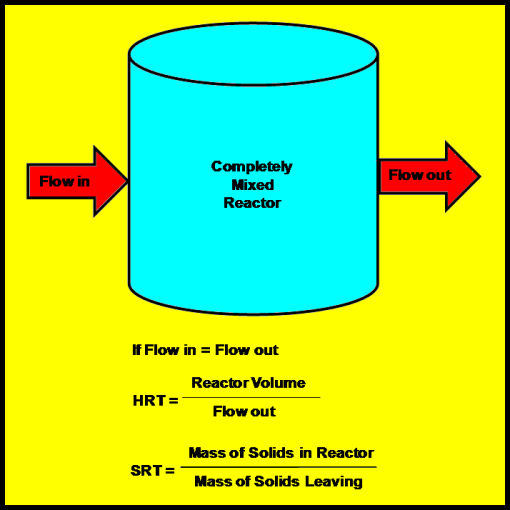
Anaerobic digestion generally takes place in a liquid, continuous flow reactor (Figure 4). If the reactor volume does not change, then flow out of the reactor equals flow into the reactor, and the average time that liquid remains in the reactor is its volume divided by the flow rate. This is called the hydraulic retention time of the reactor (HRT).
If the reactor is completely mixed, that is, the microorganisms are completely suspended in the reactor, then the time the cells remain in the reactor equals the HRT. If the HRT of a completely mixed reactor equals the reproduction time of the organisms living in the rector, a new cell is formed to replace each cell leaving the reactor, and the population within the reactor remains stable. If the HRT is shorter than the reproduction time, a new cell will not be there to replace the one leaving, and the population will decline, or “wash out.”
Solids Retention Time
Reducing HRT decreases reactor size, and smaller reactors reduce costs; therefore, many digestion systems are designed so that microorganisms remain in the reactor longer than its HRT. For instance, we could put a screen on the outlet of the reactor in Figure 4 so that some of the microorganisms are trapped inside. We can calculate cell retention time by dividing the mass of microorganisms trapped in the reactor by the mass of organisms leaving the reactor. The microbial population is kept stable by setting cell retention time equal to reproduction time.
It is easier to measure the total mass of solid particles suspended in liquid rather than the mass of viable cells; therefore, cell retention time is approximated by Solids Retention Time (SRT), or the mass of solids in the reactor divided by the mass of solids leaving.
Steady Food Supply
Microorganisms need food to reproduce and grow. Methanogens have the uncanny ability to go dormant during periods of food shortage. This is good because you can easily restart an anaerobic digester after long periods of inactivity. On the other hand, sudden increases in feeding can cause bursts of gas production, which lead to foaming and scum formation in the digester.
Temperature
Methanogens thrive in two temperature ranges. Thermophyllic (heat loving) methanogens are fast growing (reproduction time 10 to 15 days), but they operate in a fairly narrow band of temperature centered on 55 degrees C. Mesophyllic methanogens are slower growing (reproduction time up to 30 days), but they tolerate a wider range of temperatures. 35 degrees C. is optimal for mesophyllic methanogens, but they can tolerate much lower temperatures. Provided they have sufficiently long SRT, digesters operated at 20 degrees C. do not show substantial loses in gas production when compared to those operated at 35 degrees C.
Oxygen
Methanogens are strict anaerobes, meaning the least amount of oxygen is poison to them. Acid forming bacteria are more tolerant of oxygen. So, if oxygen gets into an anaerobic digester, methane concentration will drop and carbon dioxide concentration will increase in the biogas.
pH
pH is an major indicator of reactor health. A complex set of naturally occurring buffers control pH within an anaerobic digester, with organic acid-ammonia and carbonate-bicarbonate being the most important buffers. The optimum pH range for anaerobic digestion is neutral to slightly basic (pH 6.6 to 7.6).
Digester Start-up
To ensure that there is a living population of methane producing microbes in a digester, materials that contain methanogens are commonly added. When these materials come from an outside source, such as sludge from an active manure treatment lagoon or biosolids from a sewage treatment plant, it is called a “hot start.” Sometimes, digesters are brought on line with a “cold start,” meaning manure is slowly added to a liquid filled digester until gas begins to form. Hot starts are generally quicker than cold starts. Depending on digester type, a hot start can take between one and six months to bring a digester on line. It may take six months to a year for a digester to reach steady-state under a cold start.
Inhibitory Substances Commonly Found in Manure
Inhibiting elements are often called toxic, but toxicity is a misnomer. Low doses of most inhibitory agents actually stimulate biological activity. The following sets of chemicals are the major inhibitory agents found in animal manures.
Antibiotics
Antibiotics given therapeutically or fed sub-therapeutically to animals have the potential to alter the communities of microorganisms digesting manure from treated animals. The inhibitory effect of antibiotics on gas production has been shown to be minimal if high SRT is maintained and the microorganisms are given time to acclimate.
Disinfectants
Disinfectants and cleaning agents can also cause digester imbalance, although reports of upsets have not been reported in the literature. Dilution of cleaning water or bypassing the digester after cleaning is the safest tactic for handling disinfectants. Some doses may prove to have minimal effect on performance if high SRT is maintained.
Ammonia
Ammonium ion (NH4+) and its gaseous relative, Ammonia (NH3), are byproducts of protein digestion and reduction of urea. Concentrations at which Ammonical Nitrogen (NH4+NH3-N) is beneficial, inhibitory, or toxic to anaerobic digestion are given in Table 1. The toxicity of ammonia is highly dependent on pH. NH3, which is the predominant form at higher pH, is more toxic than NH4+. Usually, ammonia is not a problem in manure digesters, except for reactors treating highly nitrogenous materials such as poultry manure; and some poultry manure digesters have been able to tolerate levels as high as 6,000 mg/l if the microorganisms are given time to acclimate.
| Effect on Anaerobic Treatment | NH4+NH3-N mg/l | S– mg/l |
|---|---|---|
| Beneficial | 50-200 | <50 |
| No Adverse Effect | 200-1000 | 50-100 |
| Inhibitory at higher pH values | 1500-3000 | 100-200 |
| Toxic | >3000 | >200 |
Sulfate and Sulfide
Sulfate (SO4–) is not an inhibitory substance, per se. Its presence can reduce CH4 production because a group of bacteria called sulfate reducers can out-compete hydrotrophic methanogens for available H2. Competition with sulfate reducers does not usually reduce production from manure digesters since there is plenty of acetic acid around for acetotrophic methanogens to produce gas. The end product of sulfate reduction, Sulfide (S–), can be quite toxic to anaerobic digestion. Like ammonia, the toxicity of S– is dependent on digester pH. Stimulatory and inhibitory concentrations of sulfide are given in Table 1. Also, like ammonia, S- exists in a gaseous form, Hydrogen sulfide (H2S), so its control is a balance between source reduction, gas production, and pH.
Salt
The higher the soluble salt content in a digester, the harder microorganisms have to work in order to transport water in and out of their cells. Research has shown that it is mainly the cations (positive ions in solution) that inhibit microbial activity. Stimulatory and inhibitory concentrations of base cations are given in Table 2.
| Cation | Stimulatory (mg/l) | Moderately Inhibitory (mg/l) | Strongly Inhibitory (mg/l) |
|---|---|---|---|
| Sodium, Na+ | 100-200 | 3500-5500 | 8000 |
| Potassium, K+ | 200-400 | 2500-4500 | 12,000 |
| Calcium, Ca+ | 100-200 | 2500-4500 | 8000 |
| Magnesium, Mg+ | 75-150 | 1000-1500 | 3000 |
Precipitation of Inhibitory Substances
It should be noted that most inhibitory substances must be in solution to reduce biological activity in digesters. Therefore, the inhibitory effect of S– is reduced through precipitation of insoluble metal sulfides. Soluble Mg and NH3 are reduced by precipitation of Struvite (MgNH4PO4).
Upset Conditions and Their Control
Fermentative bacteria are generally more robust and faster growing than methanogens. The first indication that something is wrong with a digester occurs when the acid formers start to overpower the methane formers. This may show up as a drop in biogas production. First, however, the CO2 concentration in the biogas will increase, and the organic acid concentration of the reactor liquid increases. Both of these will cause a drop in pH. So, daily measurement of pH is a good method to monitor digester health. The relationship between fermentative and methanogenic communities can become so unbalanced, that even the acid formers can no longer tolerate the low pH conditions. At this point we say the digester is “stuck,” or it has “soured” or become “pickled.” Basic steps to follow in case of digester upset are:
- Reduce the feeding rate.
- Stabilize pH.
- Determine and correct the cause of the imbalance.
- Slowly increase feeding rate while maintaining neutral pH.
Sources
- Cundiff, J.S., and K.R. Mankin. 2003. Dynamics of Biological Systems. St Joseph, MI: ASABE.
- Darling, D. 2009. The Internet Encyclopedia of Science. http://www.daviddarling.info/encyclopedia/ETEmain.html. Accessed May 16, 2009
- Hamilton, D.W., P.R. Sharp, and R.J. Smith. 1985. The Operational characteristics of a manure digester for 60 beef cattle. pp 500-508, in Agricultural Waste Utilization and Management, the Proceedings of the 5th International Symposium on Agricultural Wastes. St Joseph MI: ASABE.
- McCarty, M.L. 1964. Anaerobic waste treatment fundamentals. Public Works, Sept-Dec. 1964.
- Mohr, M.A. 1983. Inhibition of methane production by ammonia nitrogen in the anaerobic digestion of poultry manure. MS Thesis. Madison, WS: University of Wisconsin.
- Ndegwa, P.M., D.W. Hamilton, J.A. Lalman, and H.J. Cumba. 2007. Effects of cycle frequency and temperature on the performance of anaerobic sequencing batch reactors treating swine waste. Bioresource Technology. 99:1972-1980
- Strauch, D. and K. Winterhalder. 1985. Effect of disinfectants, additives and antimicrobial drugs on anaerobic digestion. pp 516-522 in, Agricultural Waste Utilization and Management, the Proceedings of the 5th International Symposium on Agricultural Wastes. St Joseph MI: ASABE.
- Varel, V.H. and A.G. Hashimoto. 1982. Methane production by fermentor cultures acclimated to waste from cattle fed Monenesin, Lasalocid, Salinomycin, or Avoparcin. Applied and Environmental Microbiology. 44(6):1415.
Contributors
This document was adapted from Factsheet BAE-1747, Oklahoma State University, authored by
-
Doug Hamilton, Waste Management Specialist, Oklahoma State University with contributions from:
- Daniel Ciolkosz, Extension Associate, Penn State
- Jerry Martin II, Environmental Engineer, USDA – ARS Coastal Plains Soil, Water, and Plant Research Center
- All diagrams/images cc 3.0 Doug Hamilton, Waste Management Specialist, Oklahoma State University
Peer Reviewers
- Robert Graves, Agricultural Engineer, Pennsylvania State University
- Mark Rice, Extension Specialist Waste Management, North Carolina State University