Introduction
Woody biomass is converted into useful forms of energy (i.e. solid, liquid, or gaseous fuels) as well as useful products (e.g. polymers, bio-plastics, char, pellets, and acids) using a number of technological processes. Thermochemical processes depend on the relationship between heat and chemical action as a means of extracting and creating products and energy. This fact sheet briefly covers some of the more important thermochemical conversion and production processes used for obtaining bio-based energy and products from woody biomass.
Gasification. Gasification is a special combustion process, occurring between 1,112 and 1,832 degrees Fahrenheit, in which biomass solids are turned directly into biogas. More specifically, complex hydrocarbons of wood are decomposed into hydrogen, carbon monoxide, and carbon dioxide. Products of gasification cannot be stored easily. Consequently, the system is often integrated with other conversion processes in an effort to use the outputs in the form of various bio-based syngases. In addition to biogas, the gasification process produces ash, char, tars, methane, and other hydrocarbons. Studies have shown that gasification systems are as much as 20 percent more efficient than direct combustion systems, potentially making them more economical for power production1. (Figure 1)

Figure 1. Gasification Schematic
The chemical composition of the feedstock influences the elements in the product gas, the gasification design, and the clean-up methods that must be used. Consequently, some types of woody biomass may prove more costly to gasify than others. For example, wood residues high in sodium or potassium will require pre-cleaning prior to utilization. In general, feedstocks suitable for gasification should have a high carbon-to-nitrogen ratio, relatively low sulfur content, and low moisture content.
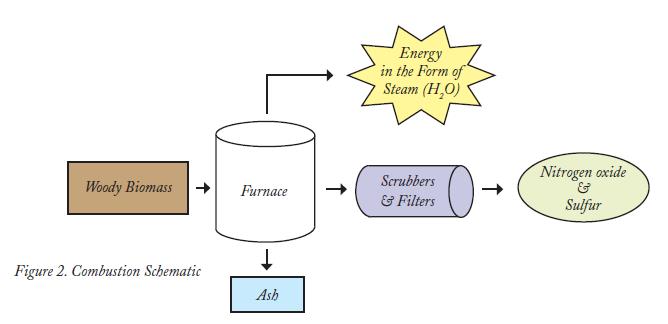
Combustion. Almost anything organic will burn. However, biomass having a low-moisture content is best suited for combustion. Combustion refers to the rapid oxidation of the feedstock as it is exposed to high heat. Most of today’s biomass-powered plants are direct-fired systems, similar to fossil fuel-powered plants. The feedstock is burned in a boiler to produce high-pressure steam that is then pumped into a turbine, over a series of blades that rotate, powering an electric generator.
Steam powered technologies have proven to be very dependable, although not always efficient. Typically, biomass-powered boilers are in the production range of 20 to 50 MWh, compared with coal-fired boilers in the production range of 100 to 1,500 MWh. Small-capacity plants generally have lower efficiencies because the equipment needed to increase energy-efficiency is not economically viable2. The most economic near-term solution is co-firing boilers with both fossil and biomass feedstocks. Much of the existing conventional power plant infrastructure can be used with little to no major modifications. (Figure 2)
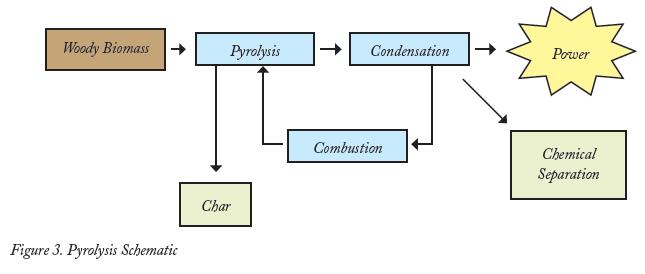
Pyrolysis. Pyrolysis is the process of rapid thermal decomposition of biomass in the absence of oxygen. This process produces energy, liquids, gases, and char. Small particles, less than a quarter inch, are delivered to a high-heat reactor where essentially no combustion occurs. The fuel must be small enough to assure high heat transfer rates during the process. At about 932 degrees Fahrenheit, the material is transformed into a vapor. In turn, it is cooled, condensed, and recollected as a liquid bio-oil. Gases that are non-condensable are recycled in a reactor for co-firing, while the char is removed for fuel or used as a commercial product. To ensure a high yield of bio-oil, the processing time from introducing the feedstock to quenching is typically less than two seconds, thus the name “fast pyrolysis.”
The primary products generated by fast pyrolysis are pyrolytic bio-oils, a combustible mixture of oxygenated hydrocarbons, and char. Reactor design and feedstock characteristics influence yield and quality. At 10 percent moisture content, biomass will yield about 150 gallons per ton. Roughly 10 to 20 percent of the product will be in the form of a non-condensable combustible gas, mainly carbon monoxide, hydrogen, and methane. It is recycled into the reactor for process heating3 (Figure 3).
Liquefaction. Thermochemical liquefaction is a process similar to what occurs in nature. Only, it occurs in minutes rather than millions of years. Hydrocarbon oils and products to produce bio-fuels are the result of subjecting intense heat and pressure to organic material. Direct liquefaction, or thermal depolymerization, has been successful in producing liquid oil, while the newer indirect liquefaction has been successful in producing syngas, ethanol, and methanol.
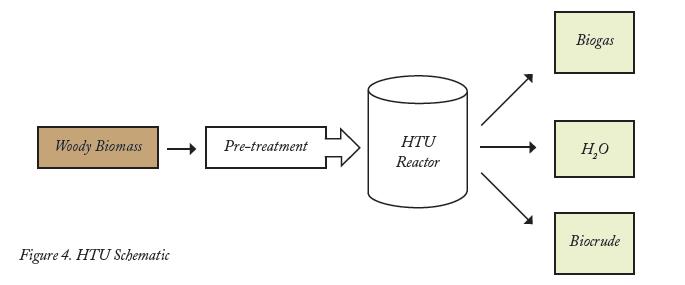
Hydrothermal Upgrading Process (HTU) . The HTU process converts a large variety of biomass feedstocks into a liquid fuel that can be upgraded to a high quality diesel fuel. The HTU process heats the feedstock in water to 572 to 662 degrees Fahrenheit at 100 to 180 bars pressure for five to 20 minutes. This facilitates the removal of oxygen. A majority of the oxygen is removed in equal parts of carbon dioxide and water (Figure 4).
The biocrude readily separates from water and can be separated into light and heavy crude through extraction. The light crude is mineral-free and can be used for high-efficiency electricity production. For large-scale applications, the light crude is upgraded to produce premium gas oil that has excellent ignition properties and can be blended directly with conventional diesel. No adaptations are needed in engines to use this fuel. The heavy crude fraction is formed as a coal-like solid that can be co-combusted for power production.
Fischer-Tropsch Process. The Fischer-Tropsch process is an established technology, first used in Germany in the 1920s. It converts carbon monoxide and hydrogen into oils or fuels that can be substituted for petroleum products. The reaction uses a catalyst based on iron or cobalt and is fueled by the partial oxidation of coal or wood-based fuels such as ethanol, methanol, or syngas typically coming from an adjacent gasifier. The current research indicates that combining woody biomass gasification processes with Fischer-Tropsch synthesis is preferred over the use of natural gas. It shows a promising route to producing economically efficient, renewable transportation fuels4. By carefully controlling the temperature and oxygen content, resulting products can range from pure syngas to “green diesel.” They can even increase the hydrogen byproduct that can be used for the production of ammonia or for fuel cells5.
For more information, please refer to the Encyclopedia of Southern Bioenergy at http://www.forestencyclopedia.com/Encyclopedia/bioenergy.
Endnotes
1 Bain, R.L.; Amos, W.A. 2003. Highlights of biopower 2003 technical assessment: State of the industry and technology. Golden, CO: National Renewable Energy Lab Report # NREL/TP-510-33502.
2 Brown, R.C. 2003. Biorenewable resources engineering: New products from agriculture. Ames, IA: Iowa State Press.
3 Agblevor, F.A.; Besler, S.; Wiselogel, A.E. 1985. Fast pyrolysis of stored biomass feedstocks. Energy and Fuels. (9): 6 p.
4 Beham, C.; Bohn, M. 1983. Analysis of a process for converting biomass to diesel fuel. Golden, CO: National Renewable Energy Lab Report # TR-252-1310.
5 USEPA. 2002. Clean alternative fuels: Fischer Tropsch. Washington DC: USEPA Report # EPA420-F-00-036.
